
In the global fight against breast cancer, Digistain stands at the forefront, offering a beacon of hope to millions. Our journey began at Imperial College London, where ground-breaking research gave birth to a technology poised to revolutionize breast cancer diagnostics. The Challenge: Every year, breast cancer affects 2.3 million individuals globally. Post-surgery, 60% of patients are advised to undergo genomic testing, an expensive and time-consuming process monopolized by a single lab in the U.S., costing around $4,000 and taking nearly two months. Consequently, only 5% undergo this vital testing. The rest, unable to access this crucial diagnostic step, often resort to chemotherapy—a treatment that, in one in four cases, is more harmful than the cancer itself. Our Solution: Digistain addresses this critical gap. Our technology transforms the current diagnostic process by enabling hospitals to analyze patient samples in-house using existing equipment, supplemented with our innovative technology. This approach offers near-instantaneous decision-making data, a stark contrast to the existing month-long waits. Central to our technology is a proprietary algorithm that identifies a unique spectral fingerprint of the tumour in the infrared spectrum, providing a personalized risk score. This score guides oncologists in selecting the most effective, individualized treatment plans, potentially reducing unnecessary chemotherapy and its harmful side effects. Impact and Potential: Our solution not only challenges the status quo in technology but also in accessibility. In a landmark government-commissioned study, Digistain demonstrated clinical evidence on par with the market leader, but at a fraction of the cost and time. Already making waves in clinical practice globally, with insurance reimbursement at leading cancer centers, our technology represents a paradigm shift in cancer care. Recognized by the Institute of Physics, the Royal Society, and Imperial College for our innovative contributions, and supported by entities like the NHS, Y Combinator, and the European Investment Bank, Digistain is more than a company—it’s a movement towards equitable, effective cancer treatment. With the potential to democratize access to life-saving diagnostics, Digistain is not just envisioning a better future for cancer patients; we are actively creating it.
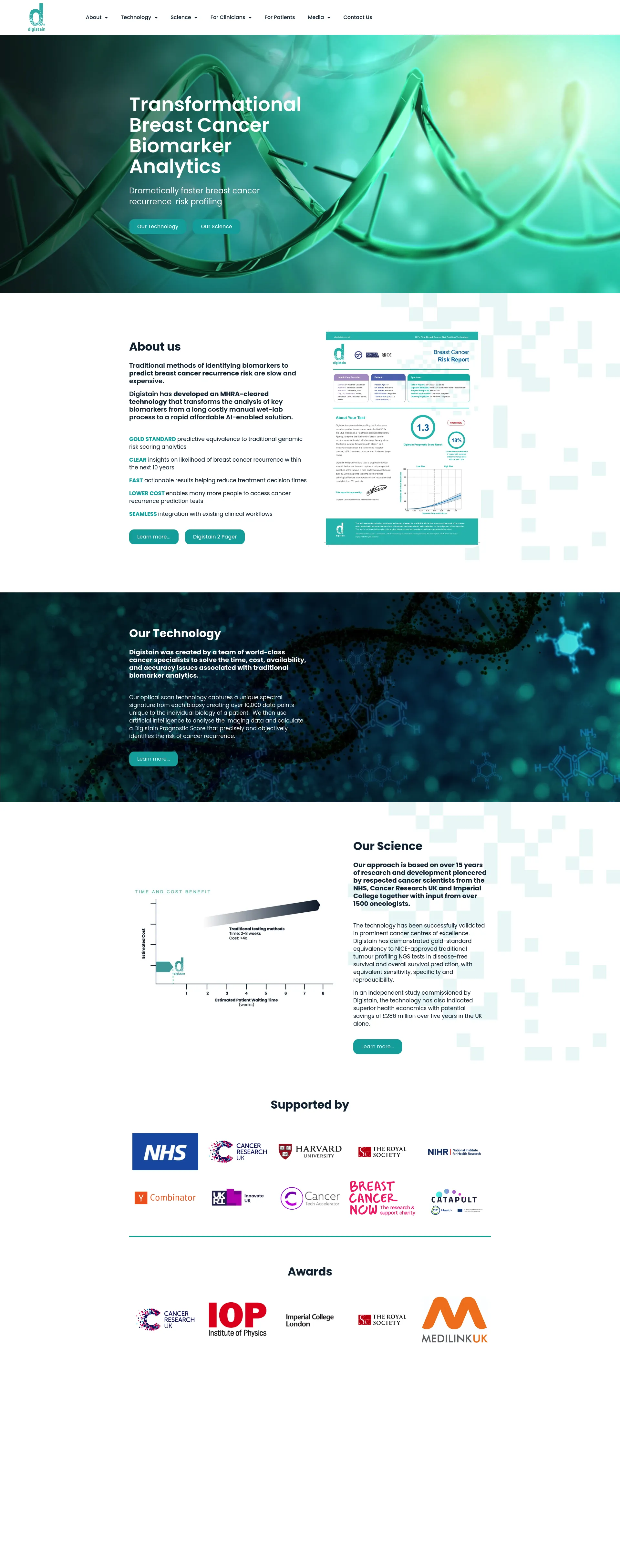
Digistain offers a revolutionary approach to breast cancer biomarker analytics, providing dramatically faster and more affordable breast cancer recurrence risk profiling.
Digistain offers competitive pricing, making advanced cancer recurrence prediction accessible to a broader population. Contact us for detailed pricing information tailored to your needs.
Digistain was developed by a team of world-class cancer specialists, including respected scientists from the NHS, Cancer Research UK, and Imperial College. Our technology has been validated in prominent cancer centers and is supported by over 1500 oncologists.
Learn more about our team and their groundbreaking work in transforming breast cancer biomarker analytics.

Tes darah sederhana untuk penyakit kompleks.

Penemuan obat yang dipersonalisasi untuk kanker darah.
Finding the right cancer drug for each patient.

Adventris kanser aşıları üretiyor.

Tes kanker .

Cofactor menutup kesenjangan pengobatan presisi dengan diagnostik prediktif

Platform pengujian cerdas yang membuka kunci kesehatan digital di rumah

Membuat skrining kanker semudah tes kehamilan

Platform pengujian genetik yang mendeteksi dan mengukur penyakit.

DaaS (Diagnostics -as-a-Service) company for India, ME and SEA

Cocokkan dengan profesional yang berpikiran sama untuk percakapan 1:1

Beralih dari Kekacauan Slack ke Kejelasan dalam Hitungan Menit

Personalisasi ribuan halaman arahan dalam waktu kurang dari 30 menit

LLM pertama untuk parsing dokumen dengan akurasi dan kecepatan

Asisten AI untuk profesional SaaS

Aplikasi panggilan telepon bertenaga AI dengan terjemahan langsung

Demo interaktif yang menyenangkan yang didukung AI—sekarang tanpa login

Kopilot Grafik Gerak AI

Lepaskan konfeti untuk menghilangkan stres & kecemasan, 100% bebas AI

Pembayaran yang Lancar untuk SaaS